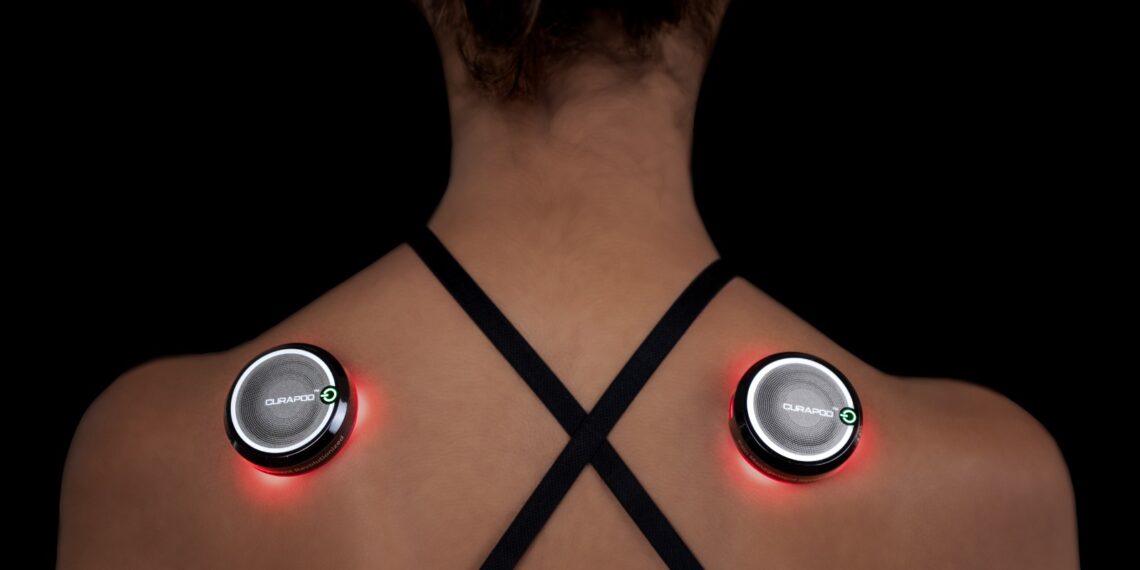
CURAPOD, a Made-in-India health-tech brand backed by Litemed India Private Limited, has received approval from the Central Drugs Standard Control Organization (CDSCO) as a Class II medical device. The approval positions CURAPOD as India’s first non-invasive, wearable device registered for personalised musculoskeletal pain management. The device is also registered with the US Food and Drug Administration, marking a key regulatory milestone for the company.
Designed for everyday use, CURAPOD targets more than 30 musculoskeletal conditions. These include chronic back and knee pain, arthritis, tendonitis, sports injuries, and routine muscle strain. The wearable is already being adopted by physiotherapy centres and wellness clinics, while early consumer uptake reflects growing interest in drug-free pain relief solutions.
The device uses medical-grade photobiomodulation technology. It delivers controlled red and near-infrared light to affected muscle and joint areas. This light-based therapy supports cellular repair, reduces inflammation, and helps speed up recovery. According to the company, many users experience measurable relief within 30 minutes of use.
CURAPOD includes an adaptive therapy engine that adjusts treatment intensity in real time. The system responds to muscle feedback and user comfort, allowing therapy to remain consistent and targeted. A companion mobile application enables users to personalise therapy programs, monitor pain levels, and track progress over time. The app also allows clinicians to review data remotely and refine treatment protocols when required.
“CURAPOD’s adaptive system adjusts intensity based on user comfort, offering consistent and targeted pain relief,” said Surya Maguluri, Founder and Chief Technology Officer of CURAPOD. “This data-driven approach allows clinicians to monitor progress remotely and deliver measurable outcomes, while making advanced therapy accessible at home.”
The wearable provides 100% drug-free therapy and is positioned as having no known side effects. It is offered with a one-year warranty and includes free expert consultation. CURAPOD is priced at ₹8,399 and can be used according to defined protocols based on pain type and intensity.
The device is currently available across India through CURAPOD’s direct-to-consumer website. The company plans to expand distribution by listing the product on major e-commerce platforms from the first quarter of 2026. Broader retail and marketplace expansion is planned over the following two years.
“By manufacturing entirely in India, we focus on trust, accessibility, and reliability,” said Sri Velliyur, Co-Founder and Chief Executive Officer of CURAPOD. “Our partnerships with physiotherapy networks and wellness chains help ensure consistent access, including in tier-2 and tier-3 cities.”